Lietuvos chirurgija ISSN 1392–0995 eISSN 1648–9942
2024, vol. 23(4), pp. 250–271 DOI: https://doi.org/10.15388/LietChirur.2024.23(4).3
Surgical Treatment and Complications of Pectus Excavatum in Childhood, Adolescence and Adulthood: A Literature Review on the Nuss Technique
Alicija Šavareikaitė
Medicinos fakultetas, Vilniaus universitetas, Vilnius, Lietuva
Faculty of Medicine, Vilnius University, Vilnius, Lithuania
El. paštas alicija.savareikaite@mf.stud.vu.lt
https://ror.org/03nadee84
Paulius Valatka
Vaikų chirurgijos, ortopedijos ir traumatologijos centras, Vaikų ligoninė,
Vilniaus universiteto ligoninė Santaros klinikos, Vilnius, Lietuva
Children’s Surgery, Orthopaedic and Traumatology Centre, Children’s Hospital,
Vilnius University Hospital Santaros Klinikos, Vilnius, Lithuania
El. paštas p.valatka@santa.lt
Abstract. Background / Objective. The Nuss procedure effectively addresses Pectus Excavatum through minimally invasive, semi-permanent insertion of metal bars, aiming to diminish the deformity and reshape the growing thorax contour. This study aims to analyze and summarize large-scale healthcare data on characteristics of complications in patients undergoing surgical correction for chest deformities, treated with the Nuss technique. Methods. A systematic review of scientific literature was performed utilizing PubMed, with the selection criteria focused on publications detailing the diagnosis and surgical treatment of Pectus Excavatum. The systematic review adhered to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines for systematic reviews. Results. According to established criteria, 404 publications were identified, of which 100 were deemed suitable to proceed with further analysis of the entire text. For the final analysis, we selected 27 articles. The patient sample size was 2,401. The average age of patients was 14.54 (ranging from 18 months to 51 years). Of all patients, distribution by gender was as follows: 511 females and 1,890 males. A total of 61 categories of complications were identified, totalling 850 complications. Intraoperative complications accounted for 1.29%, while postoperative complications accounted for 98.71%. The most common complications were pneumothorax (29.91%), displacement of the steel bar (11.32%), and pleural effusion (9.65%). Only 6 articles provided data on chest drainage, which was required for a total of 398 patients. The average hospitalization duration across all articles is approximately 7.5 days. Conclusions. While the Nuss technique effectively corrects deformities in Pectus Excavatum, postoperative complications like pneumothorax and steel bar displacement remain common. This underscores the need for meticulous patient selection and postoperative care to enhance safety and improve surgical outcomes.
Keywords: funnel chest, Pectus Excavatum, thoracoscopy, Nuss procedure, complications.
Chirurginis Pectus excavatum gydymas ir komplikacijų analizė vaikų, paauglių ir suaugusiųjų populiacijose: literatūros apie Nusso chirurginę techniką apžvalga
Santrauka. Įvadas. Nusso procedūra yra efektyvus ir minimaliai invazyvus Pectus excavatum gydymo būdas, kurio metu, siekiant sumažinti deformaciją ir pakeisti augančios krūtinės formą, naudojami metaliniai implantai. Tyrimo tikslas – išanalizuoti ir apibendrinti didelio masto sveikatos priežiūros duomenis apie pacientų, kuriems atlikta krūtinės deformacijų chirurginė korekcija, naudojant Nusso metodiką, komplikacijų ypatumus. Metodai. Sisteminė mokslinės literatūros apžvalga atlikta remiantis PubMed duomenų baze. Atrinktos publikacijos, kuriose išsamiai aprašyta Pectus excavatum diagnozė ir chirurginis gydymas. Atliekant sisteminę apžvalgą, laikytasi PRISMA (angl. Preferred Reporting Items for Systematic Reviews and Meta-Analyses) gairių. Rezultatai. Taikant nustatytus kriterijus, atrinktos 404 publikacijos, iš kurių išskirta 100 tolesnei viso teksto analizei atlikti. Galutinė publikacijų imtis – 27 straipsniai. Pacientų imties dydis – 2 401. Vidutinis amžius – 14,54 m. (nuo 18 mėn. iki 51 m.). Pasiskirstymas pagal lytį: 511 moterų ir 1 890 vyrų. Išskirta 61 komplikacijų kategorija (iš viso aptarta 850 komplikacijų). Intraoperacinės komplikacijos sudarė 1,29 proc., pooperacinės – 98,71 proc. visų komplikacijų. Dažniausios komplikacijos: pneumotoraksas (29,91 %), metalinio implanto pasislinkimas (11,32 %) ir pleuros efuzija (9,65 %). Tik šešiuose straipsniuose pateikta duomenų apie pleuros ertmės drenavimą (skirtas 398 pacientams). Vidutinė visuose straipsniuose nurodyta hospitalizacijos trukmė – apie 7,5 dienos. Išvados. Nusso chirurginė technika efektyviai koreguoja Pectus excavatum deformacijas, tačiau pooperacinės komplikacijos, tokios kaip pneumotoraksas ir metalinio implanto pasislinkimas, išlieka dažnos. Tai rodo, kad būtina kruopščiai atrinkti pacientus ir užtikrinti pooperacinę priežiūrą, siekiant padidinti saugumą ir pagerinti chirurginius rezultatus.
Reikšminiai žodžiai: įdubos krūtinė, Pectus excavatum, torakoskopija, Nusso metodika, komplikacijos.
Received: 2024-07-27. Accepted: 2024-08-28.
Copyright © 2024 Alicija Šavareikaitė, Paulius Valatka. Published by Vilnius University Press. This is an Open Access article distributed under the terms of the Creative Commons Attribution Licence, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Pectus Excavatum (PE) stands as one of the prevalent anterior chest wall deformities, affecting approximately 2–3% of the global population, with a notable predilection for males over females, occurring at a ratio of 5:1 [1]. During this deformity, a depression of the anterior wall manifests, causing the third to the seventh ribs to cave inward toward the rib-cartilage junction and/or toward the spine, resulting in a funnel-shaped appearance of the chest [2]. Common complaints and manifestations encountered in both pediatric and adult individuals with symptomatic PE include fatigue, decreased endurance, diminished exercise capacity, tachypnea with mild exertion, tachycardia, chest pain, dyspnea and palpitations [3]. In younger individuals with robust health, these symptoms may manifest during strenuous physical exertion and may be overlooked or not considered significant. It is noteworthy that symptoms often exhibit a progressive trajectory with age, and many patients may remain asymptomatic until adolescence or adulthood [4]. Additionally, it is noted that symptoms tend to escalate in accordance with the severity of PE. Kelly et al. (2007) describe that anatomically severe PE is linked to abnormal pulmonary function [5]. In literature, it is asserted that a concave chest wall configuration diminishes the volume of the pleural cavity, impeding the full expansion of the lungs during the inhalation phase of respiration. This constriction imposes heightened respiratory resistance, thereby increasing susceptibility to recurrent upper respiratory tract infections [6, 7]. In adult patients, symptom progression may become increasingly pronounced with advancing age, attributed to reduced chest wall compliance and declining compensatory mechanisms. Research conducted by Neth et al. (2011) has established a significant association between PE in elderly individuals and complaints such as dyspnea, palpitations, chronic fatigue, and manifestations such as ventricular ectopic beats [8, 9]. Patients with PE cosmetic appearance often leads to psychosocial impairments such as sense of inferiority, sense of shame, depression. There is a increasing engagement is social activities and significant improvement in body image and self-esteem of the patients that underwent chest correction surgery [10]. Zuidema et al. (2021) research provided information that prevalence of surgical correction of PE is increasing but not due to increasing incidence, but for personal’s patients wishes [11]. This shows the surgical treatment of anterior chest wall deformities necessity not only for clinical pathology, but also for aesthetics purposes. According to publication of Santaros Klinikos in Lithuania there are conducted about 30 surgical operation for sternum deformation per one year [12].
First attempt at surgical correction of PE was documented in 1911 by Meyer [13]. This technique involves removing second and third costal cartilages on the right side of the patient. In 1949, Ravitch [14] performed open intervention technique with radical mobilization of sternum and excision of the xiphisternum. In 1957 Rehbein and Wernicke published PE surgical correction technique by using crossed metal blades for stabilization [15]. Minimally invasive, semi – permanent insertion of metal bars method as an alternative to open surgery was presented in 1998, by Nuss et al [16].
The Nuss procedure involves two small incisions at the middle axillary line, with thoracoscopic control to locate the sternal depression’s deepest point. A metal introducer dissects the sternum-pericardium plane, then a tie attaches to the introducer and final bar for insertion. Under thoracoscopy, the bar is rotated 180°, moving the sternum ventrally. The procedure concludes with thoracoscopic examination to confirm the absence of lesions, lung entrapment, or bleeding. No systematic drainage or wound drains are left postoperatively, and a minor pneumothorax is benign. The metal bar remains for 2 years and is then removed under local anesthesia. The Nuss procedure eliminates the need for cartilage incisions and sternal osteotomy [2].
This study aims to analyze and summarize medical publications data on characteristics and frequency of complications in pediatrics and adulthood populations undergoing surgical correction for chest deformities, with a particular focus on PE treated with the Nuss technique.
Search Criteria and Strategy (Methodology)
Eligibility Criteria
Original studies describing different outcomes related to perioperative complications of performing the Nuss technique for PE patients were deemed eligible for review. Any type of Nuss procedure modifications, including the use of two or more trocars, one or more metal bars, and other variations, were considered. The main principle of the operative strategy required that it be described as a minimally invasive Nuss procedure. For the identification of postoperative complications, the definition provided by the National Library of Medicine was used, which identifies postoperative complications as pathological processes affecting patients after a surgical procedure. These complications may or may not be related to the disease for which the surgery was done and may or may not be direct results of the surgery. Intraoperative complications were also considered. All researches that described the procedure according to Nuss technique criteria for chest deformity treatment, specifically focusing on PE, and complications occurring during or after the procedure, up to 90 days post-surgery, were considered for inclusion. Other inclusion criteria were predefined by groups according to study tape, language, population, intervention and means of exit. Those criteria are visible in Table 1. Case series involving open (Ravitch) surgery or any other technique for PE and experiencing complications were not considered eligible. Review articles, published abstracts, letters to the editor, study protocols, and reports without full-text versions in English or Lithuanian were similarly excluded.
Information sources and search
A comprehensive literature review was conducted using the PubMed database to identify all studies published in accordance with the inclusion criteria up to January 2024. Any disagreements among the authors were resolved through consensus discussions. The search for relevant studies was performed using the following search string, limited to MeSH headings: “Funnel chest OR Pectus Excavatum”, “Thoracoscopy OR Minimally invasive surgical procedures OR Nuss procedure”; “Complications OR Intraoperative complications OR Postoperative complications”. To ensure a structured and systematic search process, a PICO table was utilized. The PICO framework included three sections describing Population, Intervention, and Outcomes, and these sections were combined using the AND operator. The Comparative section was not included in the search strategy due to the nature of the review.
Study selection
The study selection process was conducted in accordance with the PRISMA 2020 guidelines. An initial search using the specified keywords yielded 406 records from the database. Following the removal of 2 duplicate records, a total of 404 records remained for screening. These records were manually screened by titles and abstracts by the researchers. Out of these, 304 records were excluded for various reasons, including not meeting the eligibility criteria, being reports without full-text versions in English or Lithuanian, or being editorials, letters to the editor, notes, or book chapters. Full-text versions of the remaining 100 records, which were either deemed probably relevant or of unknown relevance, were subsequently obtained for further assessment (Figure 1). 17 studies could not be retrieved and were therefore excluded. The remaining 83 records were subjected to full-text analysis, which resulted in the identification of 27 articles that met all inclusion criteria and were included in the final review.
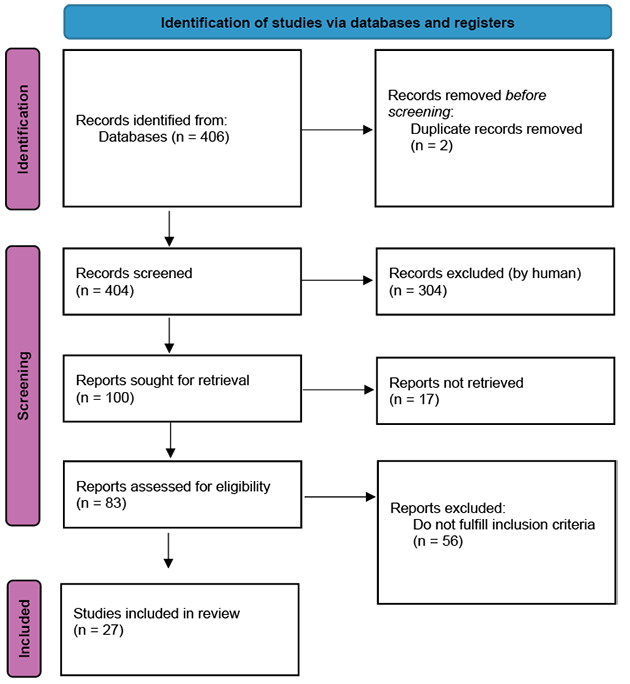
Figure 1. PRISMA 2020 flow diagram
Data systemization
After 27 articles were revied all aquired information were collected and systeminased using Microsoft Office Excel 2010. Collected information were: count of patients, median of age (if provided), intervals of age (if provided), mean of age (if provided), gender of patients count, symptoms patients felt before surgical treatment, intraoperative complications, postoperative complications, blood loss (by mililitres if provided), need for re-intervention, need for thorasic drainage after the surgery, mean of average length of hospital stay. Intraoperative complications were divided in to 7 different categories and postoperative complications in to 54 categories.
Table 1. Inclusion criteria for medical publications selection
|
Inclusion criteria for articles by study type, time period and language |
Experimental and observational studies. Scientific publications presented in English or Lithuanian. |
|
Population-based inclusion criteria |
Pediatrics and adults groups. Women and (or) men. Individuals diagnosed and surgically treated for PE. |
|
Inclusion criteria by intervention |
Patients underwent surgical thoracoscopic treatment for chest deformities using the Nuss technique. OR (AND) The patient underwent surgical treatment for early and/or late complications resulting from diagnosed and surgically (thoracoscopically using the Nuss technique) treated PE. OR (AND) The patient received therapeutic treatment for early and/or late complications resulting from diagnosed and surgically (thoracoscopically using the Nuss technique) treated PE. |
|
Inclusion criteria by means of exit |
Complications occurring during surgical treatment (subjectively and objectively) are evaluated using all instruments. Complications occurring up to 90 days after surgical treatment (surgical treatment (subjectively and objectively) are evaluated using all instruments. |
Results
Our research delves into various studies concerning medical conditions, utilizing data gathered from a comprehensive review of 27 scientific publications (Table 2). The primary types of studies within this compilation predominantly comprise retrospective designs, complemented by clinical case studies that characterize rare complications. These case studies enhance the data by outlining unique instances that larger-scale studies may overlook. There is only one prospective type article written by Felts et al. (2009). The study populations encompass mixed groups (including both children and adults) and specifically pediatrics groups. The publication years range from 2001 to 2024, showcasing a broad temporal scope that enriches the comprehension of medical advancements. Geographically, the studies cover a diverse array of countries, reflecting global contributions to medical research. These countries consist of Croatia, Japan, Norway, Republic of Korea, Spain, USA, France, People’s Republic of China, Germany, Poland, Taiwan, Finland, Netherlands, and Romania, offering a varied perspective on medical practices and outcomes across different healthcare systems and cultures.
All articles provided data on the number of patients, totalling 2,401 individuals. The gender distribution included 1,890 males (78.71%) and 511 females (21.28%), resulting in a male-to-female ratio of 3.7:1. 23 articles included information on the mean age, with an average age of approximately 14.54 years. For articles lacking mean age data, medians were included and are outlined in Table 3. One article did not furnish any data on the average age of patients. 15 articles presented information on the age range. The minimum age for PE surgery utilizing the Nuss technique was 18 months, while the maximum age recorded was 51 years.
17 articles provided data on symptoms and PE-related syndromes. One article of those, accounting for a total of 12 patients, did not offer detailed information on specific symptoms and syndromes. Among the 17 articles, comprising a total of 2,174 patients, 1,468 patients (67.52%) reported experiencing symptoms or specific syndromes. Of the reported cases (data from 16 articles), out of 1,456 patients, 1,293 patients (60.47%) reported specific symptoms (Figure 2), while 163 patients (7.62%) reported specific syndromes (Figure 3). The most common symptoms included exertional dyspnea (23.58%), exercise intolerance (18.79%), and psychosocial symptoms (15.00%). Various types of chest pain were also prevalent, collectively accounting for 10.82% of all symptoms. Among the syndromes, scoliosis (46.01%), straight back syndrome (28.22%), and Marfan syndrome (12.26%) were the most common. Less common syndromes (such as Ehlers-Danlos syndrome, congenital heart diseases, chromosome abnormalities, etc.) ranged from 0.61% to 2.45% (Figure 3).
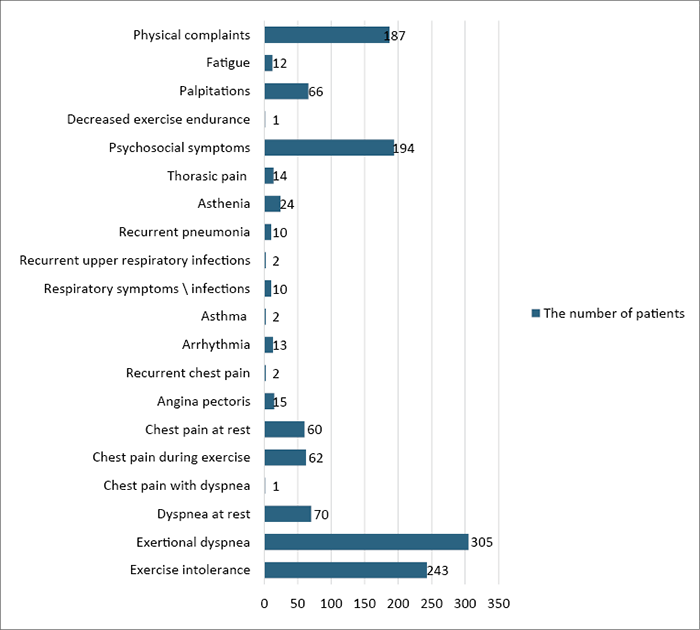
Figure 2. Distribution of symptoms
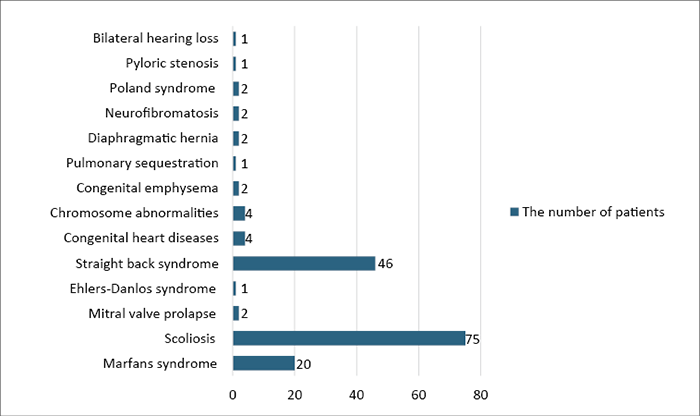
Figure 3. Distributions of syndromes
Only 7 articles provided data on intraoperative complications during PE surgical treatment (Nuss technique), totalling 11 complications across 7 categories (Figure 4). The most frequent complication was pericardial injuries (36.36%), with most instances being clinically non-significant. Hurme et al. (2008) highlighted two pericardial injuries resulting from the pericardium being caught by the tip of the introducer. Another common intraoperative complication was trocar injuries (18.18%). The overall rate of intrathoracic complications from the 7 articles (count of patient – 775) was approximately 1.42%.
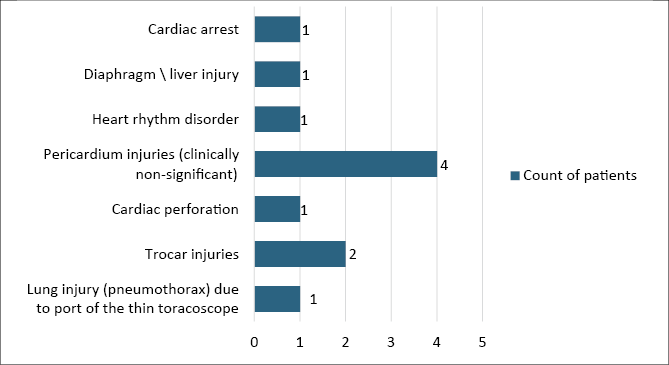
Figure 4. Distribution of intraoperative complications
Postoperative complications were documented in all 27 articles, totalling 839 reported cases across 54 categories (Figure 5). The most prevalent postoperative complication was pneumothorax (inclusive of cases resolved spontaneously, through drainage, or aspiration) at 29.91%, with spontaneously resolved pneumothorax accounting for 18.59%. Bar displacement or mobilization was a common complication, representing 11.32% of cases. Additionally, rare complications: pericarditis, superior mesenteric artery syndrome, reduced sensation and/or muscular strength, epidural catheter-related complications, bleeding, psychological symptoms, Horner syndrome, temporary paresis, intercostal nerve paresis, foreign body presence, postpericardiotomy syndrome, costal erosion, brachial plexus palsy, arteriovenous fistula, acute pulmonary edema, wound infection resulting in abscess, incomplete correction, bar flipping, and cardiac tamponade each accounted for 0.12% of cases. Requiring reintervention was highlighted in 12 articles, totalling 52 cases. The most common reason necessitating reintervention was bar rotation or bar intrathoracic migration, constituting 55.7% of cases. The need for thoracic drainage was discussed in 6 articles, totalling 398 reported cases of drainage. The calculation of reintervention and drainage incidence across all patients was precluded due to unspecified recurrent instances for individual patients.
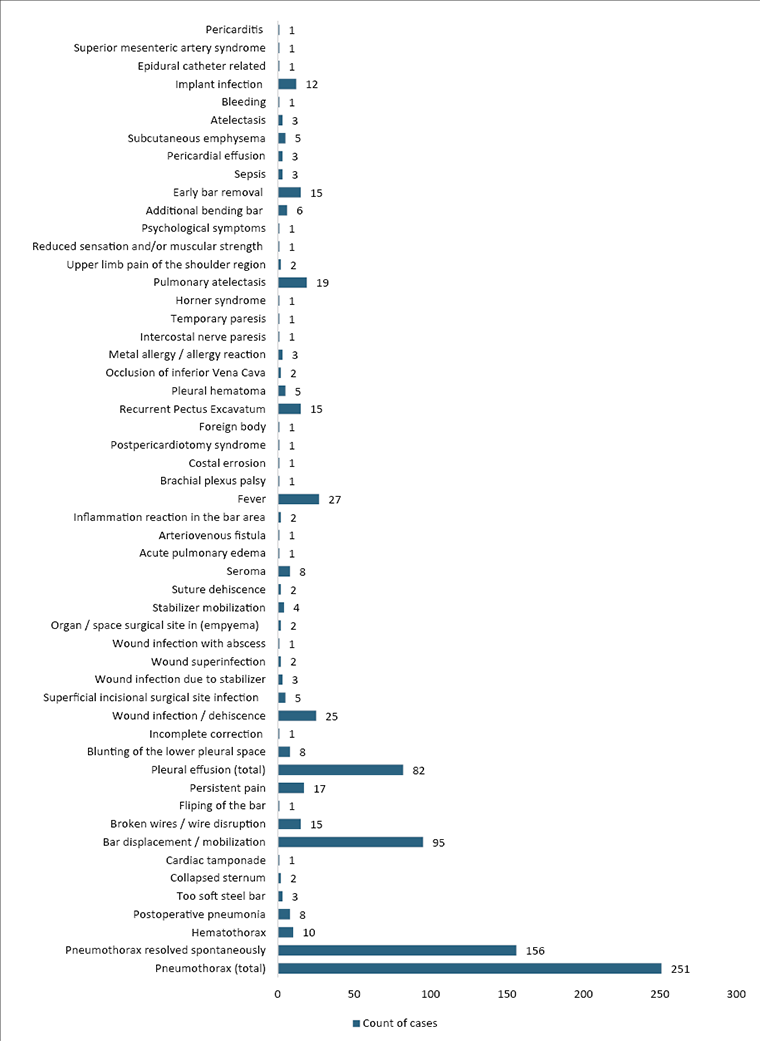
Figure 5. Distribution of postoperative complications
The complication rate was individually detailed in 10 articles (Table 4). These specific articles were selected due to inconsistencies between patient and complication counts, making it challenging to accurately calculate the complication rate. According to Kim et al. (2005), postoperative complications manifested in three patients (11.1%) in the pediatrics group (n = 27), 7 patients (58.3%) in the adolescent group (n = 12), and 7 patients (58.3%) in the adult group (n = 12). Esteva Miro et al. (2020) reported complications in 11 (35.48%) out of 31 patients, totalling 15 complications. Shah et al. (2016) noted complications in one-fourth of patients (n = 15, 24.5%), encompassing intra and postoperative issues. Pawlak et al. (2016) observed early postoperative complications in 238 patients (35.0%), with 38 in group A, 124 in group B, and 76 in group C. Kabbaj et al. (2014) of all 70 complications, categorized complication rates as early major (3/70, 4.3%), early minor (41/70, 59%), and delayed major (7/70, 4.3%) and delayed minor (5/70, 7,1%). Mao et al. reported complication rates of 14.7% in the children’s group and 37.5% in the adolescents’ group. De Loos et al. noted complications in 24% (n = 13) of adults compared to 11% (n = 30) of young participants. In the research by Chu et al., postoperative complications affected 74 patients (12.5%). David et al. reported postoperative complications in 8 out of 33 cases (24.8%). Upon examination of all the articles, it is evident that the complication rate exhibits variability, ranging from 11% in the younger cohort to 58.3% in the adult and adolescent group. This variation is contingent upon the characteristics of the complications and patient age classifications.
Table 2. Study characteristics
|
Authors |
Year |
Country |
Study period |
Population type |
Design |
|
Wu et al. |
2001 |
USA |
1996–2000 |
Pediatrics |
Retrospective |
|
Boehm et al. |
2003 |
Germany |
2002 |
Pediatrics |
NR |
|
Koichi et al. |
2003 |
Japan |
1999–2002 |
Pediatrics |
Retrospective |
|
Hoel et al. |
2004 |
Norway |
NR |
Pediatrics |
Clinical case |
|
Kim et al. |
2005 |
Republic of Korea |
1999–2003 |
Mixed |
Retrospective |
|
Žganjer et al. |
2006 |
Croatia |
2000–2005 |
Mixed |
Retrospective |
|
Hurme et al. |
2008 |
Finland |
2002–2007 |
Mixed |
NR |
|
Nath et al. |
2008 |
USA |
NR |
Pediatrics |
Clinical case |
|
Felts et al. |
2009 |
France |
2004–2009 |
Pediatrics |
Prospective |
|
Mao et al. |
2009 |
China |
2003–2008 |
Pediatrics |
Retrospective |
|
Lin et al. |
2011 |
Taiwan |
NR |
Pediatrics |
Clinical case |
|
Ballouhey et al. |
2012 |
France |
NR |
Pediatrics |
Clinical case |
|
Liu et al. |
2012 |
People’s Republic of China |
2011 |
Pediatrics |
Clinical case |
|
Ricca et al. |
2012 |
USA |
NR |
Pediatrics |
Clinical case |
|
Young Jeong et al. |
2014 |
Republic of Korea |
NR |
Pediatrics |
Clinical case |
|
Kabbaj et al. |
2014 |
France |
2004–2011 |
Pediatrics |
Retrospective |
|
Matsuoka et al. |
2014 |
Japan |
NR |
Pediatrics |
Clinical case |
|
Sakamoto et al. |
2014 |
Japan |
NR |
Pediatrics |
Clinical case |
|
Bean et al. |
2015 |
USA |
NR |
Pediatrics |
Clinical case |
|
Pawlak et al. |
2016 |
Poland |
2002–2012 |
Mixed |
Retrospective |
|
Shah et al. |
2016 |
USA |
2009–2013 |
Pediatrics |
Retrospective |
|
David et al. |
2020 |
Romania |
2007 |
Pediatrics |
Retrospective |
|
de Loos et al. |
2020 |
Netherlands |
2006–2018 |
Mixed |
Retrospective |
|
Esteva Miro et al. |
2020 |
Spain |
2010–2018 |
Mixed |
Retrospective |
|
Kim & Yong Jeong |
2020 |
Republic of Korea |
NR |
Pediatrics |
Clinical cases |
|
de Loos et al. |
2022 |
Netherlands |
2006–2018 |
Mixed |
Retrospective |
|
Chu et al. |
2024 |
Taiwan |
2006–2019 |
Pediatrics |
Retrospective |
The hospitalization period of patients who went PE surgery by Nuss technique was provided in 14 articles (13 articles provided average days period and 1 article provided median period). After calculating, the average hospitalization duration across all articles is approximately 7.5 days, it can varies from 4 days (pediatrics population) to 40 days (adults group). However, it’s important to note that this is a rough estimate, as the length of hospital stay can vary greatly depending on the specific circumstances of each case. Blood loss was mentioned in 6 articles. After calculation, the average blood loss across all articles is approximately 17.8 ml after Nuss procedure (Table 4).
Table 3. Characteristics of patients in publications
|
Authors |
Patient count / |
Mean of age / |
Intervals of age |
Symptoms / Syndroms |
|
Wu et al. |
36 patients 28 males, 8 females |
Mean age, 12.3±4.1 years |
NR |
12 patients felt syptoms. |
|
Boehm et al. |
21 patients 20 males, 1 female |
14.3 years |
From 6.3 to 16.7 years. |
NR |
|
Koichi et al. |
23 patients 21 males, |
Group 1 average, 17 years old. Group 2 average, 5 years old. |
Group 1 (teenagers) was composed of 5 males, aged from 13 to 19 years. Group 2 (younger patients) was composed of 16 males and 2 females, aged from 3 to 9 years. |
NR |
|
Hoel et al. |
1 patient 1 male, 0 females |
17 years |
NR |
NR |
|
Kim et al. |
51 patients 40 males, |
13.2±10.2 years |
From 18 months to 51 years. The pediatrics group was composed of 27 patients, with a male to female ratio of 16 to 11, and ages ranged from 18 months to 12 years (mean, 5.9±2.9 years). The adolescent group included 12 males, ranging in age from 13 to 19 years (mean, 15.8±1.9 years). The adults group were all male, with ages ranging from 20 to 52 years old (mean, 27.0±10.2 years). |
Recurrent pneumonia – 10 patients; arrhythmia – 2 patients; dyspnea on exertion – 3 patients. |
|
Žganjer et al. |
75 patients 50 males, |
NR |
From 7 years old to 20 years old. (9 patients were under 10 years old, 28 patients were between 14 and 16 years old. 1 patient was 20 years old.) |
Exercise intolerance and dyspnea – 21 patients; chest pain with exercise – 9 patients; arrhythmia – 8 patients; asthma – 2 patients; Marfans syndrome – 2 patients. |
|
Hurme et al. |
25 patients 20 males, 5 females |
14 years |
Range from 5 to 23 years. |
The symptoms recorded in the patients’ history were: exercise intolerance – 5 patients; recurrent upper respiratory infections – 2 patients; chest pain at exercise – 1 patient; minor scoliosis – 2 patients. (1 patient was deaf and was mentally disabled, one was a Jehovah’s Witness, who declined blood transfusion at the outset. 2 patients had a mitral valve prolapse by echocardiography, but had no cardiac symptoms.) |
|
Nath et al. |
1 patient 0 males, 1 female |
13 years |
NR |
NR |
|
Felts et al. |
25 patients 15 males, |
13.8 years |
From 5 to 18 years. |
Dysonea on exercise or recurrent atelectasias – 6 patients. 2 of 6 patient had a Marfans syndrome. |
|
Mao et al. |
115 patients 72 males, |
7.85±5.14 years; median, 6.5 years |
The age of the patients ranged from 2.7 to 18 years. In children group (below 12 years old) n = 75. In the adolescents group (aged 12–18 years) n = 40. |
NR |
|
Lin et al. |
1 patient 1 male, 0 females |
13 years |
NR |
NR |
|
Ballouhey et al. |
1 patient 1 male, 0 females |
15 years |
NR |
NR |
|
Liu et al. |
1 patient 1 male, 0 females |
15 years |
NR |
Decreased exercise endurance – 1 patient. |
|
Ricca et al. |
1 patient 1 male, 0 females |
14 years |
NR |
Mild scoliosis – 1 patient. |
|
Young Jeong et al. |
1 patient 1 male, 0 females |
17 years |
NR |
Exertional dyspnea – 1 patient. |
|
Kabbaj et al. |
70 patients 52 males, |
13.8 years |
Range, 6–19 years. |
Clinical respiratory symptoms in 4 patients, including 3 with Marfan syndrome and 1 with Elhers-Danlos syndrome. Scoliosis in 9 patients, including 8 with idiopathic scoliosis, and Marfan syndrome in 5 patients. |
|
Matsuoka et al. |
1 patient 1 male, 0 females |
17 years |
NR |
NR |
|
Sakamoto et al. |
2 patients 2 males, 0 females |
28.5 years |
Ranging from 17 to 23 years. |
NR |
|
Bean et al. |
1 patient 0 males, 1 female |
13 years |
NR |
Chest pain and dyspnea – 1 patient. |
|
Pawlak et al. |
680 patients 536 males, |
18.2±5.4 years |
From 7 to 49 years. The first group (Group A) consisted of 156 patients between 7 and 14 years of age (average 12.2±2.0 years). The second group (Group B) included 328 patients between 15 and 20 years of age (average 17.2±1.6 years). The oldest group (Group C) was composed of adults 21 years of age and older. In this group, the average age was 25.2±4.8 years, and age ranged from 21 to 49 years. |
Group A (patient’s count): chest pain – 1 patient; dyspnea on exertion – 59 patients; arrhythmias – 2 patients. Group B (patient’s count): chest pain – 27 patients; dyspnea on exertion – 141 patients. Group C (patient’s count): chest pain – 32 patients; dyspnea on exertion – 75 patients; arrhythmias – 1 patient. |
|
Shah et al. |
61 patients 51 males, |
15.3±3.6 years |
NR |
NR |
|
David et al. |
33 patients 27 males, |
Median 15.2 years |
Range 8–20 years. |
Symptoms were present in 21 patients: 1) effort dyspnea in 20 cases; 2) recurrent chest pain in 2 patients; 3) in 2 cases palpitations were associated to effort dyspnea; 4) clinical exam revealed different degrees of alteration of the cardiac function of in 18 patients; 5) in 5 patients (15%) Marfan syndrome was also present. |
|
de Loos et al. |
222 patients 190 males, |
Median age of 17 years |
Interquartile range, 15–20 years |
187 patients described physical complaints. |
|
Esteva Miro et al. |
31 patients 21 males, 10 females |
14.67 years |
From 4 to 27 years |
Asthenia – 24 patients; thorasic pain – 14 patients; respiratory infections – 6 patients; psycho-emotional disorders – 25 patients. |
|
Kim & Yong Jeong |
1 patient 1 male, 0 female |
17 years |
NR |
NR |
|
de Loos et al. |
327 patients 281 males, 46 females |
Young group: n = 272; median age, 16 years. Adult group: n = 55; median age, 32 years. |
Young group: n = 272; IQR, 15 to 18 years; range, 11 to 24 years. Adult group: n = 55; IQR, 27 to 38 years; range, 25 to 47 years. |
Exercise intolerance: young group – 177, adult group – 40. Psychosocial complaints: young group – 154, adult group – 15. Dyspnea at rest: young group – 58, adult group – 12. |
|
Authors |
Patient count / |
Mean of age / |
Intervals of age |
Symptoms / Syndroms |
|
Angina pectoris: young group – 5, adult group – 10. Chest pain other than angina pectoris: young group – 33, adult group – 19. Palpitation: young group – 45, adult group – 19. Fatigue: young group – 10, adult group – 2. |
||||
|
de Loos et al. |
327 patients 281 males, 46 females |
Young group: n = 272; median age, 16 years. Adult group: n = 55; median age, 32 years. |
Young group: n = 272; IQR, 15 to 18 years; range, 11 to 24 years. Adult group: n = 55; IQR, 27 to 38 years; range, 25 to 47 years. |
Exercise intolerance: young group – 177, adult group – 40. Psychosocial complaints: young group – 154, adult group – 15. Dyspnea at rest: young group – 58, adult group – 12. Angina pectoris: young group – 5, adult group – 10. Chest pain other than angina pectoris: young group – 33, adult group – 19. Palpitation: young group – 45, adult group – 19. Fatigue: young group – 10, adult group – 2. |
|
Chu et al. |
594 patients 456 males, 138 females |
The mean age at surgery was 10.0±5.0 years. |
Range, 1.7–18 years. Divided in three groups: <6 years (preschool-age), 6 to 12 years (school-age), 12 to 18 years (adolescent). |
Associated abnormalities were found in 106 patients: 1) 54 with scoliosis, 2) 46 with straight back syndrome, 3) 6 with Marfan syndrome, 4) 4 with congenital heart diseases, 5) 4 with chromosome abnormalities, 2 with congenital emphysema, 6) 1 with pulmonary sequestration, 7) 2 with diaphragmatic hernia, 8) 2 with neurofibromatosis, 9) 2 with Poland syndrome, 10) 1 with pyloric stenosis, 11) 1 with bilateral hearing loss. |
Table 4. Distribution of complications, hospitalization days and blood loss
|
Authors |
Intraoperative complications |
Postoperative complications |
Hospitalisations days |
Intraoperative blood loss (average or median) |
|
Wu et al. |
NR |
Pneumothorax – 6 cases Pneumothorax reguiring chest tube in 2 cases (from 6 cases). Pleuras effusion – 3 cases. Pectus bar displacement – 1 case. Epidural catheter – related in 1 case. Reoperation for a shifted footplate that necessitated removal – 1 case. Only 5 patients (14%) undergoing MIR required intraoperative chest tube thoracostomy, and none had drainage catheters placed. |
5.5±2.2 days |
22,1±44,7 mL |
|
Boehm et al. |
NR |
Pneumothoraces – 2 cases. Hematothorax – 1 case. Pleural effusion – 1 case. Costal errosion – 1 case. Bar dislocation – 2 cases. Postpericardiotomy syndrome – 1 case. Wound infection with abscess (requiring removal of the bar) – 1 case. |
NR |
20 mL |
|
Koichi et al. |
In Group 2 – lung injury (pneumothorax) due to the port of the thin thoracoscope (1 case). |
In Group 1 – 3 complications: fliping of the bar – 1 case; shift of the bar – 1 case. In Group 2 – 1 complication: foreign body, a buried fragment of wire behind rib – 1 case. |
Group 1 – 11±3 days. Group 2 – 7±3 days. |
No difference between the groups in terms of blood loss (Group 1 – 22±16 g, Group 2 – 15±10 g). |
|
Hoel et al. |
NR |
Cardiac tamponade 2 months after the Nuss procedure. |
NR |
NR |
|
Kim et al. |
NR |
Postoperative complications occurred in 3 patients (11.1%) in the pediatrics group, 7 patients (58.3%) in the adolescent group, and 7 patients (58.3%) in the adult group. Pediatrics patients: bar displacement – 1 case; peristent pain – 1 case. Adolescent patients: bar displacement – 1 case; peristent pain – 1 case; pleural effusion – 3 cases; incomplete correction – 1 case; pneumothorax – 1 case. Adult patients: bar displacement – 3 cases; persistent pain – 2 cases; pleural effusion – 1 case; wound infection due to stabilizer – 3 cases. The incidence of major complications requiring surgical intervention was 3.7% (bar rotation, 1 case) in the pediatrics group, 16.6% (bar rotation, 1 case; incomplete correction, 1 case) in the adolescent group, and 50% (bar rotation, 4 cases; uncontrolled persistent pain necessitating bar removal, 1 case; stabilizer-induced wound problem, 1 case) in the adult group. |
The mean duration of hospital stay was 4.9±1.5 days (range, 2 to 8), 8.0±6.3 days (range, 4 to 26), and 10.0±8.5 days (range, 4 to 40) for the pediatrics, adolescent, and adult groups, respectively (p = 0.042). |
NR |
|
Žganjer et al. |
NR |
Pneumothorax – 12 cases (8 resolved spontaneously); postoperative viral pneumonia – 6 cases; steel bar was too soft (reintervention for all) – 3 cases; collapsed sternum – 2 cases (with Marfan syndrome; reintervention was needed). |
Average 8 days. Varied from 6 to 15 days. |
Average 25 mL |
|
Hurme et al. |
Clinically non-significant pericardium puncture (were trailed with the tip of the introducer) – 2 cases. |
Intercostal nerve paresis (verified by ENMG) – 1 case. Temporary paresis or power loss of both upper limbs – 1 case. Horner syndrome (healed spontaneously) – 1 case. Residual pneumothorax (clinically non-significant) on chest radiographs – 16 cases. |
NR |
NR |
|
Asymptomatic postoperative pleural effusion – 12 cases (these effusions were probably hemothoraxes and were seen incidentally on routine chest radiograms). Blunting of the lower pleural space – 8 cases. Larger postoperative hemothorax (with visible fluid level in pleural space) – 3 cases. Dislocation of the bar (with needed correction) – 1 case. Dislocation of the bar (without correction) – 2 cases. Dislocations of the bar (remote clinically non- Wound infections occurred – 2 cases (one was superficial and healed conservatively while the other appeared 2 months after the operation). Postoperative pneumonia – 2 cases. Pulmonary atelectasis (was seen radiologically) – 19 cases. Resistant upper limb pain of the shoulder region – 2 cases. Reduced sensation of the right shoulder and numbness and reduced muscular strength of the right upper limb – 1 case. Severe mental and psychological symptoms (depression, lack of facial expression in face, withdrawal and distress) – 1 case. |
||||
|
Nath et al. |
NR |
Acute occlusion of the inferior vena cava – 1 case. |
NR |
NR |
|
Felts et al. |
NR |
Postoperative minimal pneumothorax (resolved spontaneously) – 3 cases. Inflammation reaction in the bar area (a small aseptic fluid collection, with no bacteria found on bacteriological samples taken) – 2 cases. Secondary implant displacement (required early surgical revision on the 15th postoperative day) – 1 case. Fever (two days after discharge; blood tests demonstrated Staphylococcus aureus) – 1 case. Thorasic drain was necessary in 14 patients. |
NR |
NR |
|
Mao et al. |
NR |
In children group the rate of complications was 14.7. In adolescents group the rate of complications were 37.5. Early postoperative: 1) subcutaneous emphysema (children group – 3; adolescents group – 2), 2) pneumothorax with spontaneous resolution (children group – 2; adolescents group – 2), 3) pneumothorax requiring aspiration (children group – 1; adolescents group – 1), 4) pneumothorax requiring chest tube (children group – 0; adolescents group – 1), 5) hemopneumothorax (children group – 1; adolescents group – 1), |
In children group (below 12 years old) (n = 75) the length of stay 7.2±1.5 days. In the adolescents group (aged 12–18 years) |
NR |
|
6) atelectasis (children group – 1; adolescents group – 2), 7) pericardial effusion (children group – 1; adolescents group – 1). Late complications included: 1) bar displacement (children group – 1; adolescents group – 2), 2) seroma and infection (children group – 1; adolescents group – 3), 3) recurrence occurred in 2 (1.8%). |
||||
|
Lin et al. |
NR |
Bilateral hemothorax – 1 case. |
7 days |
During the operation, only about 10 mL of blood loss was noted. |
|
Ballouhey et al. |
NR |
Mechanical occlusion of inferior vena cava – 1 case. |
NR |
NR |
|
Liu et al. |
NR |
Injury of the right brachial plexus (brachial plexus palsy). |
7 days |
NR |
|
Ricca et al. |
NR |
Superior mesenteric artery syndrome – 1 case. |
NR |
NR |
|
Young Jeong et al. |
Cardiac perforation and delayed development of hypovolemic shock after successful Nuss procedure |
NR |
NR |
NR |
|
Kabbaj et al. |
Pericardial contusion (with no clinical effects) – 1 case. Heart rhythm disorder (resolved immediately with ephedrine therapy) – 1 case. |
Of the 44 early complications, 3 (3/70, 4.3%) were major: 1) 2 pleural effusions, drained on days 4 and 6, respectively; 2) 1 acute sepsis treated with surgical drainage and antibiotic therapy. 41 (41/70, 59%) were minor: 1) 37 cases of pneumothorax (<15 mm with spontaneous resolution); 2) 1 pericardial effusion (resolved with NSAID therapy); 3) 1 staphylococcal chest wall sepsis treated with antibiotics; 4) 2 cases of pneumonia (treated with antibiotics). Of the 8 delayed complications, 3 (3/70, 4.3%) were major: 1) 1 secondary post-traumatic displacement of the implant (surgical revision); 2) 1 chest wall sepsis after implant removal; 3) 1 premature implant removal due to an allergic reaction. 5 (5/70, 7.1%) were minor – 5 patients with pain at the tip of the implant that resolved after implant removal. A chest drain was used in 36 patients, for a mean duration of 1.5 days (range, 1–5 days). |
Hospital stay length for the repair procedure was 7.2 days (range, 5–12 days). |
NR |
|
Matsuoka et al. |
NR |
Simultaneous bilateral spontaneous |
NR |
NR |
|
Sakamoto et al. |
NR |
1. Fever – 2 cases. 2. Bilateral pleural effusion – 2 cases. 3. Metal allergy – 2 cases. |
NR |
NR |
|
Bean et al. |
NR |
Arteriovenous fistula between the left internal mammary artery and the pulmonary venous system. |
NR |
NR |
|
Pawlak et al. |
NR |
Early postoperative complications, were observed in 238 (35.0%) patients and occurred in 38 patients from Group A, 124 patients from Group B, and 76 patients from Group C. Number of cases of complications when all groups are combined: 1) pneumothorax – 157 (spontaneous resolution – 104; drainage – 52); 2) pleural effusion – 52 (spontaneous resorption – 10; chest tube or thoracocentesis – 42); 3) pleural hematoma – 5; 4) rotation of the bar – 17; 5) fever – 24; 6) recurrence of deformation – 12. Routine chest drainage: Group A – 60 patients, Group B – 137 patients, Group C – 91 patients. |
NR |
NR |
|
Shah et al. |
2 intraoperative trocar injuries |
Complications occurred in 1–4 of patients (15, 24.5%). Complications related to bar fixation and position – 6 cases. Broken wires – 5 cases (3 requiring an operative procedure – two because of pain, the third for a late pneumothorax (one month postoperatively)). 3 cases of broken wires (not caused overt problems or symptoms) were found incidentally at the time of bar removal at the end of the period of surgical fixation. A sixth patient required re-positioning of bar migration. Postoperative wound infections – 3 cases. Severe pain – 2 cases: 1) with a large postoperative pleural effusion – 1 case; 2) recurrent PE – 1 case. |
5.8±2.1 days |
NR |
|
David et al. |
NR |
Postoperative complications occurred in 8 of the 33 cases (24.8%): 1) wound-related, with dehiscence or infection – 4 cases; 2) postoperative pleural effusions – 3 cases (in 2 of them spontaneous resolution after conservative treatment occurred and in 1 case needle aspiration was performed); 3) pneumothorax occurred 24 hours after surgery – 1 case (pleural drainage was necessary); 4) pericarditis – 1 case. Reoperations were necessary in 5 cases, 4 for closing the dehiscent surgical wound and 1 pneumothorax drainage. |
Hospital stay ranged from 6 to 40 days, mean 12 days. |
NR |
|
de Loos et al. |
NR |
Complications occurred in 20 participants (9%): 1) superficial incisional SSI – 5; 2) deep incisional SSI – 0; 3) organ / space SSI (empyema) – 1; 4) pneumonia – 2; 5) pneumothorax requiring intervention – 1; 6) pleural effusion requiring intervention – 0; 7) reoperation for bleeding – 0; 8) additional bending bar – 3; 9) displacement requiring intervention – 6; 10) early bar removal – 2. SSI – surgical-site infection. |
Median (IQR) 4 (4–5) days (counted for all three surgeons). |
The median amount of blood loss showed a narrow range from 5 to 10 mL, whereas the maximum 75th percentile was 10 mL. |
|
Esteva Miro et al. |
NR |
Complications were noted in 11 patients (total of 15 complications were recorded). Stabilizer mobilization – 4 cases; suture dehiscence – 2 cases; seroma – 4 cases; bar mobilization – 2 cases; acute pulmonary edema – 1 case; wound superinfection – 2 cases. Of the aforementioned patients, 6 required re-intervention (2 – bar intrathorasic migration; 3 patients – bar detachment, 1 – wound dehiscence). 2 patients required thorasic drainage. |
Mean hospital stay was 7.9 days (range, 5–16 days). |
NR |
|
Kim & Yong Jeong |
Cardiac arrest during the Nuss procedure |
NR |
4 days |
NR |
|
de Loos et al. |
NR |
Complications occurred in 24% (n = 13) of adults compared with 11% (n = 30) of young participants, with only the incidence of minor complications significantly higher among adult participants (young, 4% vs. adult, 11%). Major complications (young group – 20, adult group – 7): 1) bar displacement requiring reoperation young group – 7, adult group – 2; 2) additional bar bending young group – 3, adult group – 0; 3) bar removal within 3 years for chronic pain young group – 6, adult group – 4; 4) bar removal for any other cause within 1 year young group – 1, adult group – 1; 5) reoperation for bleeding young group – 1, adult group – 0; 6) pneumothorax requiring intervention young group – 1, adult group – 0; 7) empyema young group – 1, adult group – 0. Minor complications young group – 10, adult group – 6: 1) wound infection young group – 6, adult group – 0; 2) pneumonia young group – 2, adult group – 2; 3) chronic pain without bar removal young group – 2, adult group – 4. |
Length of hospital stay, d, median (IQR). Young group – 5.0 (4.0–6.0) days. Adult group – 5.0 (4.0–6.0) days. |
NR |
|
Chu et al. |
Pericardial injury – 1 case. Diaphragm / liver injury – 1 case. |
Postoperative complications occurred in 74 patients (12.5%). Postoperative complication was: 1) bar displacement (n = 41); 2) implant infection (n = 12); 3) wound infection (n = 10); 4) wire disruption (n = 10); 5) pneumothorax (n = 6); 6) hemothorax (n = 3); 7) pleural effusion (n = 2). |
The typical length of hospital stay was 6 days (including surgery, postoperative intensive care unit stay for 1 day, respiratory training, rehabilitation prescriptions, and discharge preparations). |
Discussion
Surgical treatment
According to a study by Zganjer et al. (2006), the traditional Ravitch surgical procedure for correcting chest deformation pathology, which involves cartilage resections, sternal osteotomy, and sternal fixation, has shown successful outcomes. However, this method has several drawbacks, including prolonged operating time, increased blood loss, potential impairment of rib growth, visible thoracic scars, and extended hospitalization duration [17]. Conversely, minimally invasive procedures offer several advantages, such as the absence of an anterior chest wall incision, minimal visibility of scars, reduced operating time, minimal blood loss, and early resumption of normal activities.
The Nuss Procedure, a minimally invasive surgical technique, has gained widespread acceptance globally for the correction of PE. According to a survey of 74 respondents, 42% (31 out of 74) of surgeons have adopted this minimally invasive approach as their preferred method for PE repair [18]. Despite this, follow-up data in the adult population regarding the Nuss procedure is limited [19].
Patient selection for PE surgical treatment is debatable. It is generally recommended for young patients between the ages of 6 and 12 years, as suggested by Croitoru et al. (2002) [20]. However, the application of this method in adolescents and adults has been progressively increasing. In fact, a study by Park et al. (2004) [21] reported a 21.2% procedure rate in PE patients older than 15 years of age. Overall, the best candidates for the Nuss operation are young patients with a symmetrical deformity [22].
While minimally invasive techniques such as the Nuss Procedure are becoming more popular among older patient populations, long-term follow-up data remain limited, particularly in adults. Therefore, careful consideration should be given to each case to ensure the most appropriate and effective treatment. In addition to conventional methods of treating PE, several alternative approaches have been introduced recently, including silicone implant reconstruction, the magnetic mini-mover procedure, and the vacuum chest wall lifter [23–25].
Our literature review indicates that the majority of researched articles combined pediatrics populations (pediatrics and mixed populations ratio 2.86:1). The minimum age for PE surgery utilizing the Nuss technique was 18 months, while the maximum age recorded was 51 years.
Complication rates
In the reviewed literature, the complication rate was described in 10 articles. These articles show variability in complication rates, ranging from 11% in younger cohorts to 58.3% in adult and adolescent groups. It is important to note that the complication rate depends on various factors, including patient characteristics, surgical modifications, medications, and postoperative hospitalization. A study by Tikka et al. (2016) found that patients with PE who experienced postoperative complications had a higher likelihood of recurrence, especially those with major complications. Univariate analysis determined that complications were the only statistically significant factor associated with PE recurrence [26].
Regarding the Nuss Procedure for PE, Kim et al. (2005) found a higher incidence of complications in older patients, including bar displacement, persistent pain, and reoperation. These complications were largely connected to the application of the procedure in the adult population [27]. In de Loos et al. (2020), chronic pain unresponsive to medical treatment and urging bar removal was classified as a major complication (CDC IIIb) and occurred in 2% (n = 6) of young patients and 7% (n = 4) of adult participants [28]. However, a study by Chu et al. (2024) showed that postoperative complications were most frequently observed in the preschool-age group (14.3%), followed by the school-age group (11.9%) and adolescent group (11.5%), with no significant difference in postoperative complication rates among the three groups (p = 0.665). Regarding rare complications such as hemothorax, several studies confirmed that the risk of postoperative hemothorax associated with the Nuss procedure is less than 1% (30,31).
A study by Nuss et al. (2002) discusses the complication rates of the Nuss Procedure for PE. They report that the incidence of bar displacement ranges from 4% to 8%, with efforts made to decrease this rate. The introduction of a bar stabilizer by Nuss reduced the incidence of bar displacement from 15.7% to 5.4%. Combining a wiring technique with the stabilizer further reduced the incidence, although this technique led to wound issues near the stabilizer due to pressure damage [28].
Risk factors for complications
One discussed risk factor for complications of the Nuss technique is the force required for sternum elevation during the operation. In research by Fonkalsrud et al. (2002), it was noted that the force necessary to elevate the sternum varies with age. For children under 10 years old, the elevation force is reported to be 15 lbs (approximately 6.80 kg). For adolescents and adults, the mean force required is approximately 32 lbs (14.51 kg) and 41 lbs (18.60 kg), respectively [16]. This higher force not only causes considerable pain but also increases the likelihood of bar displacement. Ohno et al. (2003) compared the results of the Nuss procedure by age and concluded that patients older than 13 years experienced more complications and had poorer cosmetic results than younger patients [29].
Operational risk factors related to the surgeon’s experience, movements inside the thoracic cavity, and the number of metal bars used are crucial for both intraoperative and late postoperative complications. Zou et al. (2017) encountered cardiac arrest twice during a modified Nuss procedure, when pulling the pectus bar and after rotating the bar, in patients with PE [30]. Various techniques have been used to avoid cardiac injury during the Nuss procedure [31–33]. Lifting the sternum is key to releasing the substernal space for the pectus bar insertion. Devices such as crane and wire sutures, lifting hooks, the Kent or Langenbeck retractor, and the Vacuum Bell device have been used for this purpose.
Another important factor mentioned in the literature is asymmetry. According to Park et al. (2010), major complications after the Nuss procedure were associated with the severity of asymmetry (eccentric long canal type / Grand Canyon type) and the lack of surgeon experience. In adults with severe asymmetrical deformity, the Ravitch procedure may be a better initial choice than the obstinate application of the Nuss procedure due to the high probability of bar displacement and associated complications [34, 35].
Trauma and exercise may also be significant risk factors for intra and postoperative complications. Trauma can increase friction between the bars and ribs, becoming a risk factor for late-onset hemothorax after the Nuss procedure. In a clinical case by Lin et al. [36], late-onset bilateral hemothorax resulted from friction between the implants and internal mammary vessels, causing life-threatening hemothorax with hypovolemic shock.
Significance of literature review and limitations
The results of this literature review encompass 24 years of practice and advancements in the surgical treatment of chest deformities in the pediatrics and adults population using the Nuss technique. Both intraoperative and postoperative complications have been identified. These findings may benefit medical professionals by providing insights into the most common and rare perioperative complications associated with this procedure. This information could facilitate improved patient selection, more precise procedure planning, and enhanced methods for managing complications. Furthermore, these results can be used to offer patients and their families more comprehensive information regarding the procedure’s safety, effectiveness, and potential complications.
The findings may also stimulate further research in this area to gain a deeper understanding of the procedure’s effects and potential long-term outcomes. Future research may include larger and longer studies that assess long-term patient outcomes and complication rates.
However, the study sample may not be sufficiently representative or may be limited by the number of patients from certain demographic groups, potentially restricting the generalizability of the conclusions. Additionally, essential information about the patients or the course of the procedure, necessary for broader conclusions about the pathogenetic mechanisms of the complications, is missing. Risk factors for complications have not been identified or evaluated.
Conclusions
Surgical correction of thoracic deformities, particularly PE, using the Nuss technique can be effective, safe, and successful, though it carries certain risk factors for complications.
The most common intraoperative complication is pericardial injury (36.36%), with most instances being clinically non-significant. The most common postoperative complications are pneumothorax (29.91%) and bar displacement or mobilization (18.59%). Therefore, accurate planning of the procedure and careful patient selection are crucial to reducing the likelihood of complications. According to ten articles, the complication rate varies significantly, ranging from 11% in the younger cohort to 58.3% in the adult and adolescent group.
References
1. Abdullah F, Harris J. Pectus excavatum: more than a matter of aesthetics. Pediatr Ann 2016; 45(11): e403–e406.
2. Brochhausen C, Turial S, Müller FKP, Schmitt VH, Coerdt W, Wihlm JM, Schier F, Kirkpatrick CJ. Pectus excavatum: history, hypotheses and treatment options. Interact Cardiovasc Thorac Surg 2012; 14(6): 801–806.
3. Mansour KA, Thourani VH, Odessey EA, Durham MM, Miller Jr JI, Miller DL. Thirty-year experience with repair of pectus deformities in adults. Ann Thorac Surg 2003; 76(2): 391–395; discussion 395.
4. Jaroszewski DE, Fonkalsrud EW. Repair of pectus chest deformities in 320 adult patients: 21 year experience. Ann Thorac Surg 2007; 84(2): 429–433.
5. Kelly Jr RE, Shamberger RC, Mellins RB, Mitchell KK, Lawson ML, Oldham K, Azizkhan RG, Hebra AV, Nuss D, Goretsky MJ, Sharp RJ, Holcomb 3rd GW, Shim WKT, Megison SM, Moss RL, Fecteau AH, Colombani PM, Bagley TC, Moskowitz AB. Prospective multicenter study of surgical correction of pectus excavatum: design, perioperative complications, pain, and baseline pulmonary function facilitated by internet-based data collection. J Am Coll Surg 2007; 205(2): 205–216.
6. Hu YZ, Wei FK, Luo QC. Sternal elevation operation on pectus excavatum: report of 6 cases. Chinese Journal of Pediatric Surgery 1987; 8: 341.
7. Zou X, Lin Y, Jin H, Cai S, Xu X, Yin W, Geng Q, Chen J, Liang B, He J, Li W. Screening for pectus excavatum among primary students and establishment of a pectus excavatum screening program in Dongguan, China. J Thorac Dis 2015; 7(5): 868–874.
8. Kragten HA, Siebenga J, Höppener PF, Verburg R, Visker N. Symptomatic pectus excavatum in seniors (SPES): a cardiovascular problem? A prospective cardiological study of 42 senior patients with a symptomatic pectus excavatum. Neth Heart J 2011; 19(2): 73–78.
9. Abid I, Ewais MM, Marranca J, Jaroszewski DE. Pectus excavatum: a review of diagnosis and current treatment options. J Am Osteopath Assoc 2017; 117(2): 106–113.
10. Fortmann C, Petersen C. Surgery for deformities of the thoracic wall: no more than strengthening the patient’s self-esteem? Eur J Pediatr Surg 2018; 28(4): 355–360.
11. Zuidema WP, van der Steeg AFW, Oosterhuis JWA, van Heurn E. Trends in the treatment of pectus excavatum in the Netherlands. Eur J Pediatr Surg 2021; 31(3): 261–265.
12. Vilniaus universiteto ligoninė Santaros klinikos. Available at <https://www.santa.lt>.
13. Meyer L. Zur chirurgischen Behandlung der angeborenen Trichterbrust. Berl Klin Wschr 1911; 48: 1563–1566.
14. Ravitch MM. The operative treatment of pectus excavatum. Ann Surg 1949; 129(4): 429–444.
15. Rehbein F, Wernicke HH. The operative treatment of the funnel chest. Arch Dis Child 1957; 32(161): 5–8.
16. Nuss D, Kelly Jr RE, Croitoru DP, Katz ME. A 10-year review of a minimally invasive technique for the correction of pectus excavatum. J Pediatr Surg 1998; 33(4): 545–552.
17. Zganjer M, Zupancić B, Popović L. A 5-year experience of a minimally invasive technique for correction of pectus excavatum in Croatia. Acta Medica (Hradec Králové) 2006; 49(2): 105–107.
18. Hebra A, Swoveland B, Egbert M, Tagge EP, Georgeson K, Othersen Jr HB, Nuss D. Outcome analysis of minimally invasive repair of pectus excavatum: review of 251 cases. J Pediatr Surg 2000; 35(2): 252–257.
19. Coln D, Gunning T, Ramsay M, Swygert T, Vera R. Early experience with the Nuss minimally invasive correction of pectus excavatum in adults. World J Surg 2002; 26(10): 1217–1221.
20. Croitoru DP, Kelly Jr RE, Goretsky MJ, Lawson ML, Swoveland B, Nuss D. Experience and modification update for the minimally invasive Nuss technique for pectus excavatum repair in 303 patients. J Pediatr Surg 2002; 37(3): 437–445.
21. Park HJ, Lee SY, Lee CS, Youm W, Lee KR. The Nuss procedure for pectus excavatum: evolution of techniques and early results on 322 patients. Ann Thorac Surg 2004; 77(1): 289–295.
22. Jacobs JP, Quintessenza JA, Morell VO, Botero LM, van Gelder HM, Tchervenkov CI. Minimally invasive endoscopic repair of pectus excavatum. Eur J Cardiothorac Surg 2002; 21(5): 869–873.
23. Margulis A, Sela M, Neuman R, Buller-Sharon A. Reconstruction of pectus excavatum with silicone implants. J Plast Reconstr Aesthet Surg 2006; 59(10): 1082–1086.
24. Harrison MR, Estefan-Ventura D, Fechter R, Moran Jr AM, Christensen D. Magnetic mini-mover procedure for pectus excavatum: I. Development, design, and simulations for feasibility and safety. J Pediatr Surg 2007; 42(1): 81–85.
25. Schier F, Bahr M, Klobe E. The vacuum chest wall lifter: an innovative, nonsurgical addition to the management of pectus excavatum. J Pediatr Surg 2005; 40(3): 496–500.
26. Tikka T, Kalkat MS, Bishay E, Steyn RS, Rajesh PB, Naidu B. A 20-year review of pectus surgery: an analysis of factors predictive of recurrence and outcomes. Interact Cardiovasc Thorac Surg 2016; 23(6): 908–913. DOI: 10.1093/icvts/ivw263.
27. Kim DH, Hwang JJ, Lee MK, Lee DY, Paik HC. Analysis of the Nuss procedure for pectus excavatum in different age groups. Ann Thorac Surg 2005; 80(3): 1073–1077. DOI: 10.1016/j.athoracsur.2005.03.070.
28. Chu CC, Chang JW, Yang HH, Kuo FC, Tsai HL. Outcomes of the Nuss procedure in children with pectus excavatum: 14 years of experience. J Chin Med Assoc 2024; 87(3): 314–319. DOI: 10.1097/JCMA.0000000000001054.
29. Ohno K, Morotomi Y, Ueda M, Yamada H, Shiokawa C, Nakaoka T, Tsujimoto K, Nakahira M, Moriuchi T, Harumoto K, Yoshida T. Comparison of the Nuss procedure for pectus excavatum by age and uncommon complications. Osaka City Med J 2003; 49(2): 71–76.
30. Zou J, Luo C, Liu Z, Cheng C. Cardiac arrest without physical cardiac injury during Nuss repair of pectus excavatum. J Cardiothorac Surg 2017; 12: 61. DOI: 10.1186/s13019-017-0624-2.
31. Park HJ, Jeong JY, Jo WM, Shin JS, Lee IS, Kim KT, Choi YH. Minimally invasive repair of pectus excavatum: a novel morphology-tailored, patient-specific approach. J Thorac Cardiovasc Surg 2010; 139(2): 379–386.
32. Takahashi T, Okazaki T, Yamataka A, Uchida E. Usefulness of Kent retractor and lifting hook for Nuss procedure. Pediatr Surg Int 2015; 31(11): 1103–1105.
33. Togoro SY, Tedde ML, Eisinger RS, Okumura EM, de Campos JRM, Pêgo-Fernandes PM. The Vacuum Bell device as a sternal lifter: an immediate effect even with a short time use. J Pediatr Surg 2018; 53(3): 406–410.
34. Park HJ, Lee SY, Lee CS. Complications associated with the Nuss procedure: analysis of risk factors and suggested measures for prevention of complications. J Pediatr Surg 2004; 39(3): 391–395.
35. Shah M, Frye R, Marzinsky A, Phillips MR, Adamson W, McLean SE. Complications associated with bar fixation after Nuss repair for pectus excavatum. Am Surg 2016; 82(9): 781–782.
36. Lin CW, Chen KC, Diau GY, Chu CC. Late-onset vital complication after the Nuss procedure for pectus excavatum. Pediatr Surg Int 2012; 28(1): 71–73. DOI: 10.1007/s00383-011-2936-y.
37. de Loos ER, Pennings AJ, van Roozendaal LM, Daemen JHT, van Gool MH, Lenderink T, van Horck M, Hulsewé KWE, Vissers YLJ. Nuss procedure for pectus excavatum: a comparison of complications between young and adult patients. Ann Thorac Surg 2021; 112(3): 905–911. DOI: 10.1016/j.athoracsur.2020.10.017.
38. Nuss D. Croitoru DP, Kelly Jr RE, Goretsky MJ, Nuss KJ, Gustin TS. Review and discussion of the complications of minimally invasive pectus excavatum repair. Eur J Pediatr Surg 2002; 12(4): 230–234.
39. Vegunta RK, Pacheco PE, Wallace LJ, Pearl RH. Complications associated with the Nuss procedure: continued evolution of the learning curve. Am J Surg 2008; 195(3): 313–316 (discussion 316–317).
40. Fonkalsrud EW, Reemtsen B. Force required to elevate the sternum of pectus excavatum patients. J Am Coll Surg 2002; 195(4): 575–577.